Discover the game-changing science behind EndeavorRx
EndeavorRx is the first and only FDA-authorized prescription video game to treat inattention in ADHD.
Created by world-class neuroscientists and award-winning game designers, it targets areas of the brain that play a key role in attention function.
A transformative approach
It’s a digital therapeutic. Designed to improve attention, EndeavorRx targets brain regions associated with interference processing, focus, and multitasking. Your patient must filter out distractions and challenge their motor skills.
It’s adaptive. Patented Selective Stimulus Management Engine (SSME™) technology adjusts game difficulty in real-time. As your patient performs better, the game becomes harder. This challenge is central to the therapeutic effects of the intervention.
It’s clinically effective, safe, and durable. EndeavorRx demonstrated statistically significant improvements in several measures of ADHD-related symptoms in kids 8-17 with ADHD. Patients saw sustained benefit during a 4-week treatment pause and increased benefit with a second month of treatment.²
Learn How to Prescribe¹ Kollins SH et al Lancet Digital Health 2020; ² STARS Adjunct Trial, 2021
Clinically proven
After 1 month of treatment:¹
-
56% of parents reported improved attention.
-
48% of kids had statistically significant improvement in ADHD-related impairments.
After 2 months of treatment:²
-
75% of kids said they could more easily pay attention.
-
68% of parents reported improvements in ADHD-related impairments.
In all EndeavorRx clinical trials, 0% of participants reported serious adverse events.³
Learn More About Efficacy
¹ Kollins SH et al Lancet Digital Health 2020; ² STARS Adjunct Trial, 2021; ³Instructions For Use, 2023

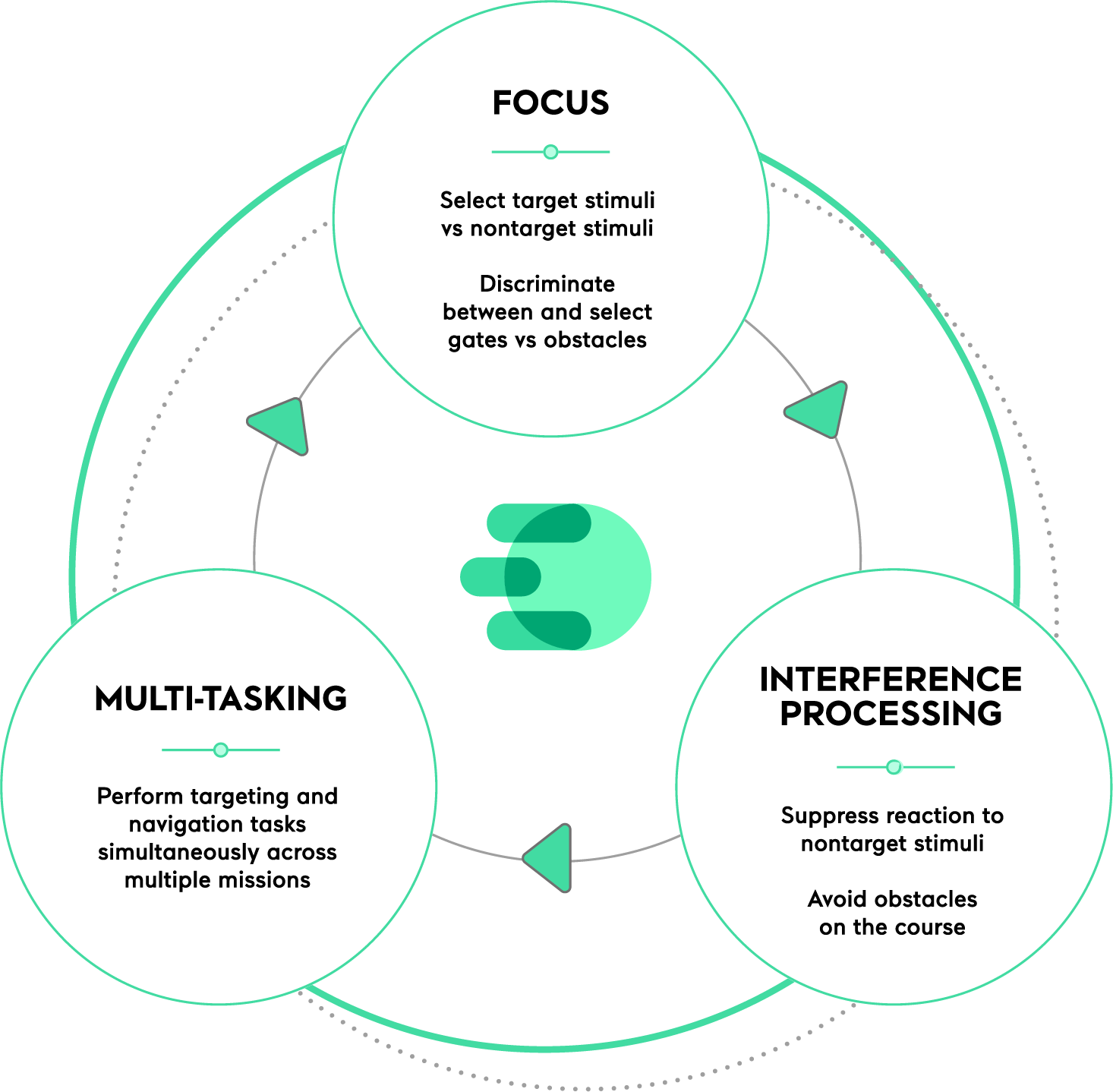
EndeavorRx strengthens three elements of attentional control
EndeavorRx strengthens focus, interference processing, and multitasking. The game presents sensory stimuli and simultaneous motor challenges that target neural systems in the brain related to attention.
Successfully navigating each level requires the focus to both manage multiple tasks and filter out distractions. As your patient performs better, the game becomes harder. This real-time algorithm works to increasingly challenge and strengthen their attentional control.
At a high level, this is our Selective Stimulus Management System (SSME™) in action – the highly responsive technology behind EndeavorRx’s gameplay design.
Play your medicine
EndeavorRx is prescribed just like a medication – with a specific dose. The recommended prescribed play time for all patients is approximately 25 minutes a day, 5 days a week, over at least 4 consecutive weeks.³
While EndeavorRx does not have prescribed “strengths” for different patients, the game’s difficulty will adapt according to their progress. As long as your patient is playing consistently and trying their best, they’re engaging with the treatment as intended. Learn How to Prescribe
³ Instructions For Use, 2023
Follow their treatment journey with EndeavorRx Insight
Our free companion app for parents connects directly to their child’s EndeavorRx treatment, so they can get a daily snapshot of their in‑game progress.

-
Mission completion
Parents can check‑in to see if their child has completed all of their play for the day.
-
Daily effort
By analyzing in‑game interactions, we calculate a daily score to give parents a sense of their child’s level of effort and if their child is playing with the correct rules.
-
Get support
Having an issue? The app can help connect parents to our support team.
Download FREE
EndeavorRx:
Indications, Safety and Cautions
Indications:
EndeavorRx is a digital therapeutic indicated to improve attention function as measured by computer-based testing in children ages 8-17 years old with primarily inattentive or combined-type ADHD, who have a demonstrated attention issue. Patients who engage with EndeavorRx demonstrate improvements in a digitally assessed measure, Test of Variables of Attention (TOVA®), of sustained and selective attention and may not display benefits in typical behavioral symptoms, such as hyperactivity. EndeavorRx should be considered for use as part of a therapeutic program that may include clinician-directed therapy, medication, and/or educational programs, which further address symptoms of the disorder.
Safety:
No serious adverse events were reported. Of 342 participants who received AKL-T01 in the two clinical trials supporting EndeavorRx authorization for age ranges 8-17, 17 participants (4.97%) experienced treatment-related adverse events (TE-ADE) (possible, probable, likely). TE-ADEs reported at greater than 1% across the studies include: frustration tolerance decreased (2.34%) and headache (1.17%). Other adverse events occurred less than 1% and included dizziness, emotional disorder, nausea, and aggression. All adverse events were transient and no events led to device discontinuation. Across other studies in children and adolescents with ADHD, rates of adverse events were similarly low (<10%) and no Serious Adverse Events have been reported. All reported adverse events across all clinical trials resolved at the end of treatment. Users should consider the totality of evidence presented along with their health care provider when considering incorporating AKL-T01 into their treatment plan.
Cautions:
Rx only: Federal law restricts this device to sale by or on the order of a licensed health care provider. EndeavorRx should only be used by the patient for whom the prescription was written. For medical questions, please contact your child’s healthcare provider. If you are experiencing a medical emergency, please dial 911. EndeavorRx is not intended to be used as a stand-alone therapeutic and is not a substitution for your child’s medication.
If your child experiences frustration, emotional reaction, dizziness, nausea, headache, eye-strain, or joint pain while playing EndeavorRx pause the treatment. If the problem persists contact your child’s healthcare provider. If your child experiences a seizure stop the treatment and contact your child’s healthcare provider.
EndeavorRx may not be appropriate for patients with photo-sensitive epilepsy, color blindness, or physical limitations that restrict use of a mobile device; parents should consult with their child’s healthcare provider.
Please follow all of your mobile device manufacturer’s instructions for the safe operation of your mobile device. For example, this may include appropriate volume settings, proper battery charging, not operating the device if damaged, and proper device disposal. Contact your mobile device manufacturer for any questions or concerns that pertain to your device.